The cotton genetic improvement team of the National Key Laboratory of Crop Genetic Improvement and the protein science research team recently worked together to analyze the structure of the cotton cellulose synthase(GhCesA7), making a breakthrough in the related field. In this study, mammalian expression system and cryo-electron microscopy were used to analyze the homotrimer structure of GhCesA7, and it was found that the conformation of GhCesA7 was mainly stabilized by TM7 and PCR domains. Meanwhile, the in vitro activity of GhCesA7 was demonstrated by Isotopic labeling method in vitro, revealing the molecular mechanism of the synthesis of sugar chains by GhCesA7.
Cellulose is a polysaccharide polymer composed of β-1, 4-glucan, which is the oldest and most abundant natural polymer on the earth and the most valuable renewable resource for human beings. Cellulose is also the main component of plant cell walls, accounting for more than 50% of the carbon content in the plant kingdom. Cotton fiber is the most important natural textile raw material. The cellulose content in the secondary wall of cotton fiber can reach more than 90%. Cellulose in the cell wall of higher plants is synthesized by cellulose synthase (CesA) localized on the plasma membrane. Cellulose synthase is a large multi-subunit complex. The structure of cotton cellulose synthase and the mechanism of cellulose synthesis from the perspective of structural biology are helpful to understand the secondary wall deposition process of cotton fiber and design breeding to improve fiber quality. "Analyzing the structure of cellulosic synthase and thus optimizing the activity of cellulosic synthase could improve the regeneration efficiency of biomass energy," the researchers said.
Cellulose synthase (CesA) belongs to GT-2 (Glycosyltransferase Family 2) type, which is distributed in higher plants and a few bacterium such as Rhodobacter. The structure of bacterial Cellulose Synthase (Bcs) has been reported in previous studies, and its mechanism is relatively clear. However, only the CesA structure of poplar has been reported in higher plants.
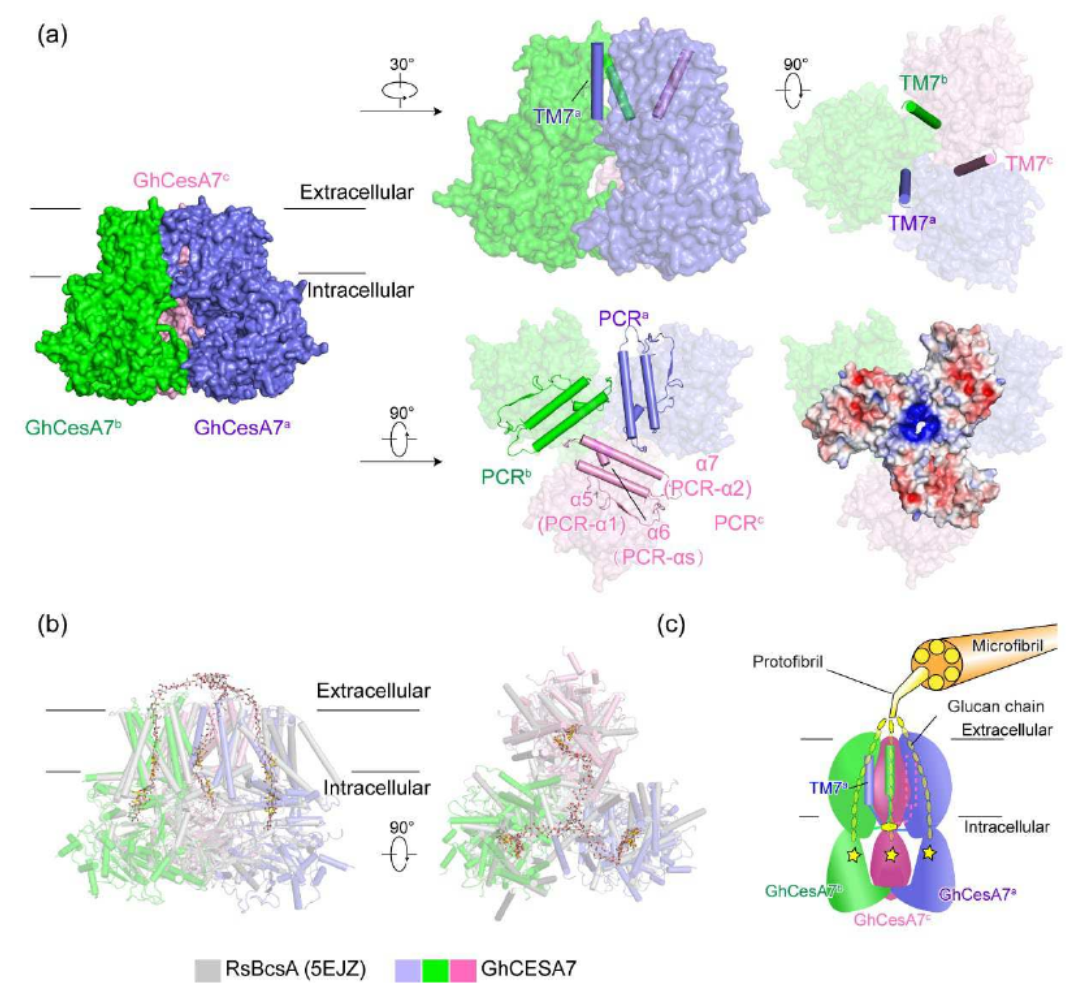
In this study, based on the reference genomes of island cotton and upland cotton drawn by the cotton floral genetic improvement team (Nature Genetics, 2019), sequences and expression information of all 34 CesAs were obtained, and codon optimization was performed to screen the expression system of mammalian expression, thereby obtaining the GhCesA7 protein and successfully analyzing its homotrimer structure. The key domain of TM7 and PCR-stabilized trimer conformation was found, while The previous eight transmembrane domains based on biological information were actually only seven transmembrane in the structure and the predicted transmembrane domain 5 (TM5) was actually an Interface Helix (IF3). Each trimer has a sugar chain visible in it. Further comparison with the bacterial BcsA-BcsB complex revealed that some amino acids, such as aspartic acid, glutamine, tryptophan, which have great influence on activity, are conserved in both eukaryotes and prokaryotes.
In the experiment of cellulose synthase activity, the CesA activity was significantly reduced by mutation of aspartic acid at 540, aspartic acid at 742, and tryptophan at 784. CesA is expressed in vitro in the form of homotrimer, but it may be responsible for cellulose synthesis in vivo in the form of heterotrimer. This study analyzed the structure of cellulose synthase in cotton and laid a solid foundation for further analysis of the assembly of CesA heteropolymer and the structure of super complex.
Translated by:Cai Luyang
Source from:http://news.hzau.edu.cn/2021/0301/59475.shtml
Abstract:
Cellulose is one of the most abundant organic polymers in nature. It contains multiple β‐1,4‐glucan chains synthesized by cellulose synthases (CesAs) on the plasma membrane of higher plants. CesA subunits assemble into a pseudo‐6‐fold symmetric cellulose synthase complex (CSC), known as a ‘rosette complex’. The structure of CesA remains enigmatic. Here we report the cryo‐EM structure of the homotrimeric CesA7 from Gossypium hirsutum at 3.5‐angstrom resolution. The GhCesA7 homotrimer shows a C3 symmetrical assembly. Each protomer contains seven transmembrane helices (TMs) which form a channel potentially facilitating the release of newly synthesized glucans. The cytoplasmic glycosyltransferase domain (GT domain) of GhCesA7 protrudes from the membrane, and its catalytic pocket is directed towards the TM pore. The homotrimer GhCesA7 is stabilized by the transmembrane helix 7 (TM7) and the plant conserved region (PCR) domains. It represents the building block of CSCs and facilitates microfibril formation. This structure provides insight into how eukaryotic cellulose synthase assembles and provides a mechanistic basis for the improvement of cotton fiber quality in the future.
Paper link:https://onlinelibrary.wiley.com/doi/abs/10.1111/pbi.13571